

The noble gases are colourless, odourless, tasteless, nonflammable gases. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).

We can determine the number of neutrons as #14-6=8# neutrons.Įxample 2. noble gas, any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table. Of these, six isotopes 78 Kr, 80 Kr, 82 Kr, 83 Kr, 84 Kr and 86 Kr occur naturally.
KRYPTON MASS NUMBER PLUS
56Naturally occurring krypton is made of five stable isotopesand one (78 Kr ) which is slightly radioactivewith an extremely long half-life, plus traces of radioisotopesthat are produced by cosmic raysin the atmosphere. Krypton has 33 known isotopes with mass numbers ranging from 69 to 101. Jump to main content Periodic Table Home History Alchemy Podcast Video Trends Periodic Table Home History Alchemy Podcast Video Trends You do not have JavaScript enabled. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. How many neutrons are in the nucleus of a chromium atom To determine this, you would subtract as shown: (3.4.2) 52 24 28 neutrons in a chromium atom The composition of any atom can be illustrated with a shorthand notation called A/Z format. There are 34 known isotopesof krypton(36Kr) with atomic mass numbersfrom 69 through 102. Element Neon (Ne), Group 18, Atomic Number 10, p-block, Mass 20.180. Now write the isotopic notation for carbon-14. Atoms of the element chromium ( Cr) have an atomic number of 24 and a mass number of 52.
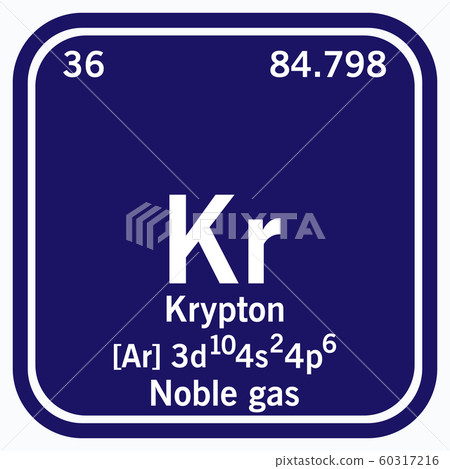
The name carbon-14 tells us that this isotope's mass number is #14#. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.Įxample 1: What is the isotopic notation for the isotope carbon-14?įrom the periodic table, we see that the atomic number (number of protons) for the element carbon is #6#. However, because different isotopes have different numbers of neutrons, they can differ in mass number, which is the sum of the protons and neutrons in the nucleus. 14 6 C We can determine the number of neutrons as 14 6 8 neutrons. Now write the isotopic notation for carbon-14. The name carbon-14 tells us that this isotopes mass number is 14. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. All atoms of the same element have the same number of protons, which is the atomic number of that element. From the periodic table, we see that the atomic number (number of protons) for the element carbon is 6. Krypton (from Ancient Greek:, romanized: kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei.


 0 kommentar(er)
0 kommentar(er)
